In the historical narrative of healthcare technology, the “clipboard” has long been a symbol of systemic inefficiency. For decades, the patient’s first interaction with a provider was defined by a stack of photocopied forms, redundant data entry, and dense, impenetrable legalese. However, as we move through 2026, the industry is witnessing a fundamental architectural shift. We are moving past simple “digital forms” toward a sophisticated clinical workflow automation model known as Consent-as-a-Service (CaaS).
As a technology partner in the healthcare space, we recognize that intake is no longer just an administrative hurdle: it is the strategic front line of patient engagement and clinical data integrity.
In this article, we’re going to explore the evolution of CaaS, the infrastructure required to build it, and why this technology is now the cornerstone of modern clinical workflow automation.
Key insights
While many organizations have automated scheduling, the consent stage remains a manual, static bottleneck that triggers administrative fatigue and patient churn.
This article explores the transition to Consent-as-a-Service (CaaS) – an API-first, FHIR-native architectural layer that transforms legal intake from a one-time “clipboard” event into a dynamic, “agentic” service.
Why manual clinical intake and “static” consent (PDFs, e-signatures) are no longer sustainable
For years, the front door of healthcare—patient scheduling—has been the industry’s most visible friction point.
Aren’t we all familiar with the “scheduling chaos”:
- the endless phone tag,
- the staff juggling three priorities at once,
- and the provider calendars filled with gaps and double-bookings
But this chaos is only a symptom of a much larger systemic shift.
The healthcare industry is facing a “triple threat”: record-high labor costs, a shrinking workforce, and an aging population requiring more complex care.
According to the American Hospital Association (AHA) 2026 Health Care Workforce Scan, administrative burden remains a top driver of clinician burnout, contributing to over 50% of reported fatigue cases; solving this requires a deeper commitment to comprehensive clinical workflow automation.
As we move into 2026, a new and more complex bottleneck has emerged. Even when a clinic successfully automates the appointment through predictive scheduling and digital reminders, the entire workflow often grinds to a halt at the consent stage.
While automated scheduling gets the patient to the door, the traditional static consent process (like PDFs and simple e-signatures) creates an administrative tax that we’ve seen many small-to-mid-sized healthtech firms can no longer afford to pay.
When consent is treated as a manual, one-off task rather than a service, it introduces 3 existential risks:
- Data fragmentation: Currently, 19% of organizations lack a “single point of truth” for patient consent. Without a centralized Consent-as-a-Service (CaaS) model, permission data is trapped in silos, creating massive compliance risks under GDPR and evolving national data protection laws.
- The friction paradox: Today’s patients expect the same instant, digital access they experience in retail and banking. In fact, 77% of patients now expect to complete all intake and consent forms digitally before they even arrive. A static PDF that is difficult to open on a mobile device or requires a physical signature isn’t just an inconvenience; it’s a reason for patient churn.
- The legal illiteracy: A 2025 study revealed a staggering reality: 76.3% of informed consent forms present low readability scores, often requiring a university-level education to comprehend. In a 2026 regulatory environment, “uninformed consent” is a ticking legal time bomb.
What is agentic consent? And Consent-as-a-Service (CaaS)?
In the previous era of healthtech, “digital consent” usually meant a chatbot that could prompt a user to sign a PDF. In 2026, we are moving beyond the chatbot and into the realm of agentic AI.
Unlike standard conversational AI, which simply follows a script, an agentic consent system is an autonomous entity designed to sit at the heart of your clinical workflow automation strategy. It doesn’t just ask for a signature; it understands the intent and context of the clinical trial or procedure. These agents can:
- Verify compliance in real-time: Instantly check a patient’s location and profile against a matrix of global regulations (GDPR, HIPAA, or India’s DPDP) to ensure the correct legal disclosures are presented.
- Explain, not just inform: Use Natural Language Processing (NLP) to translate complex legal jargon into plain language. If a patient asks, “What happens to my data if I leave the study?” the agent provides a legally accurate, simplified answer on the fly.
- Trigger downstream workflows: The moment consent is granted, the agent doesn’t just store a file; it pings the pharmacy to ship a kit, updates the EHR, and notifies the lab—all without human intervention.
The new consent process: from Documents to Dialogue
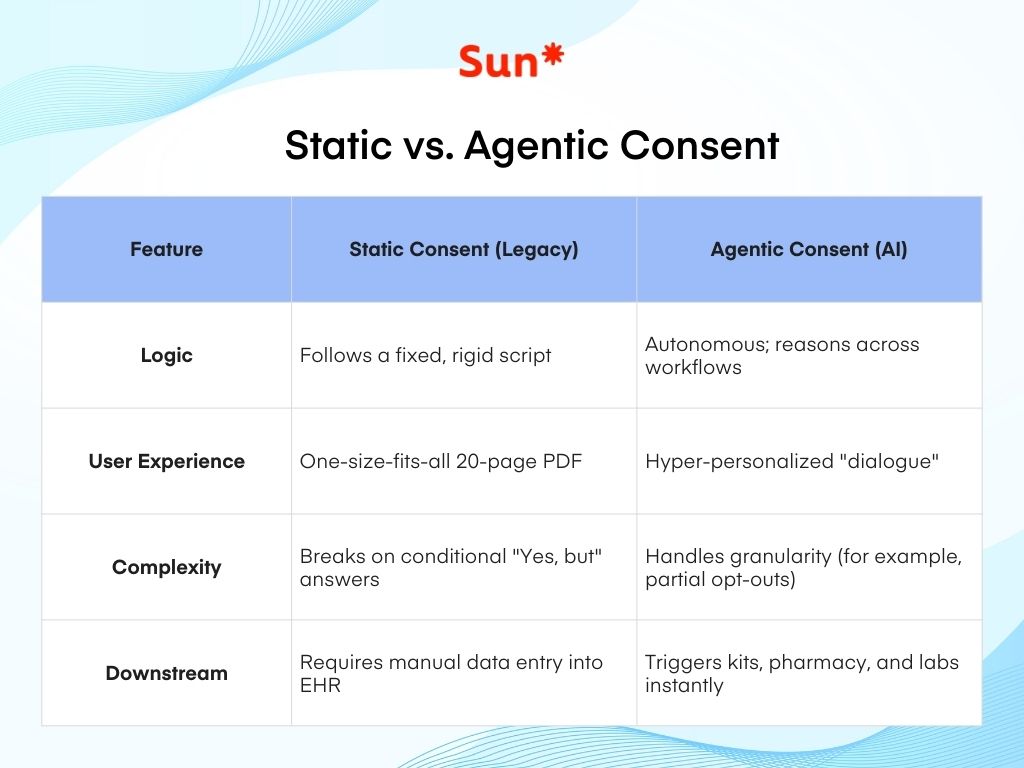
For decades, clinical intake has been a defensive “Terms of Service” exercise – think of the 20-page document designed to protect the institution.
Agentic consent transforms this into a high-trust, interactive engagement.
By breaking the 20-page document into bite-sized, interactive modules, the system ensures the patient is actually “informed.”
This isn’t just a UX improvement. You can see it as a new legal safeguard. When the consent process is a dialogue rather than a hurdle, patient trust increases, and the likelihood of “uninformed consent” (a major legal risk) plummets.
Clinical workflow automation focus: handling the nuance
Traditional automation is fragile—it fails the moment a patient gives a conditional answer like ‘Yes, but only for this part.’
Agentic systems, however, thrive on granularity.
If a patient consents to blood work but opts out of genetic sequencing, the workflow doesn’t just stop. The AI dynamically adjusts the instructions for that specific patient, ensuring the lab only receives what was permitted. This keeps the clinical process moving while respecting the patient’s choices—something a static PDF simply cannot do.
What is Consent-as-a-Service (CaaS)?
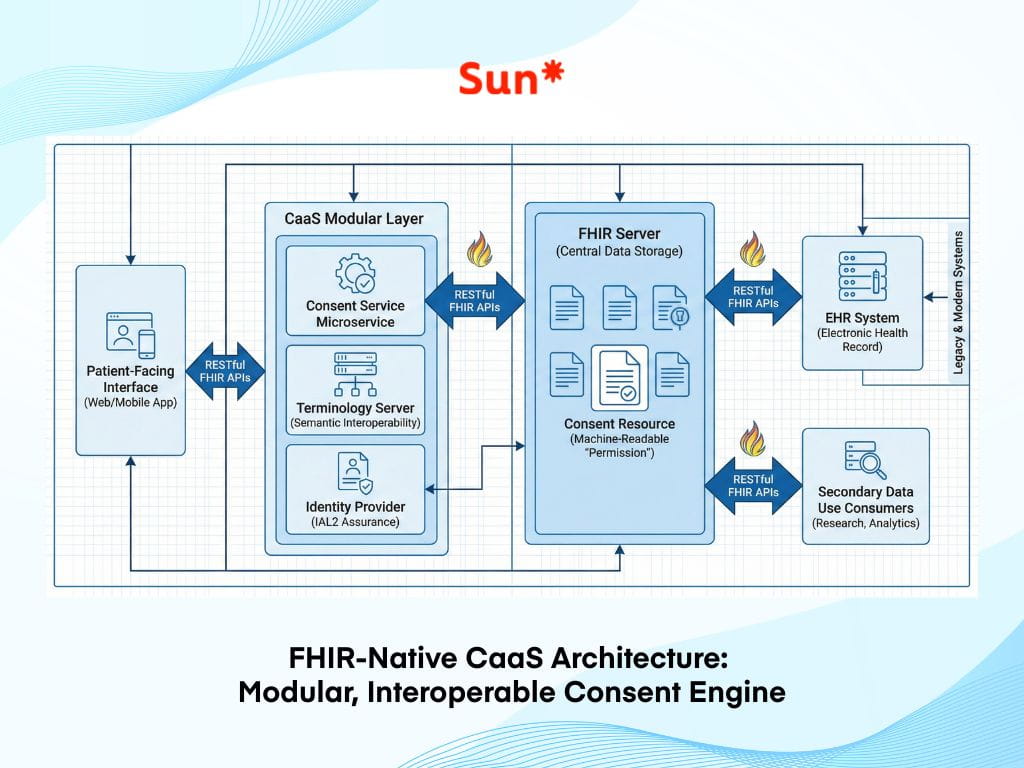
To power these agentic interactions, the underlying infrastructure must change. CaaS is not a standalone app; it is a modular, API-first layer that sits between the patient-facing interface and the Electronic Health Record (EHR).
The goal of CaaS is to kill the clipboard by transforming consent from a one-time event into a living service.
By 2026, the market for patient experience technology is projected to reach $32.33 billion, driven largely by software that can automate these complex legal and clinical workflows.
Key architectural components of CaaS include:
- FHIR-native integration: Leveraging HL7 Fast Healthcare Interoperability Resources (FHIR) to ensure that consent preferences aren’t trapped in a PDF but are shared across the care continuum as machine-readable data.
- Identity assurance (IAL2): Implementing high-level identity verification (such as passkeys or mobile driver’s licenses) to ensure that digital signatures are legally unassailable and “non-repudiable.”
- AI-driven simplification: Using NLP to act as a bridge between the legal team and the patient, ensuring that a “Grade 6” reading level is maintained across all intake touchpoints.
What are the ROIs for clinical workflow automation?
For small and mid-sized healthtech companies, investing in clinical workflow automation is no longer just a luxury but a fundamental necessity for survival in a competitive market. Implementing consent as a service goes far beyond simply meeting legal requirements. It serves as a way to unlock significant financial and operational returns.
Reducing the administrative burden
Traditional intake processes are essentially a “tax” on your staff’s time. Research for 2026 still shows that manual paperwork is the single biggest cause of clinical delays.
One of the most immediate benefits of agentic consent and CaaS is the reduction of the administrative burden that traditional intake processes place on clinical staff.
By adopting an automated service, organizations can reduce the time it takes to complete patient intake by as much as 70%. When the system handles the explanation of risks and the collection of signatures automatically, patients move from initial interest to full enrollment in minutes rather than days.
This efficiency prevents patients from dropping out of the process and ensures that clinical trials or specialty clinics operate at maximum capacity.
2. Advantage of agility over legacy
Smaller healthtech firms often have a unique advantage over industry giants in this area. While larger corporations may have more resources, they are frequently slowed down by old software and rigid internal structures.
Mid-sized players are typically more agile and can adopt new technologies without the need to overhaul decades of legacy code. This flexibility allows a smaller firm to integrate a modern consent API and reach market readiness in a matter of months, whereas a larger competitor might spend years on the same transition.
In the current landscape, this speed to market is often more valuable than a massive research budget.
3. Benefits of the secondary use of data
Automating consent creates long-term value through the secondary use of data.
Historically, using patient data for new research years after the initial collection was a missed opportunity because it required tracking down patients to ask for permission again. An automated service maintains an active legal connection with the patient through a process known as re-consent.
If the initial agreement was captured through a digital service, the platform can reach out for new permissions automatically as new research opportunities arise. This approach transforms a single data point into a lasting asset that can be legally shared or used for future innovation without the need for constant manual intervention.
Challenges and ethical considerations in automated consent
The challenge of automated explanations
While the benefits of automating these workflows are significant, moving toward a system that relies on AI and autonomous services introduces several complicated challenges.
One of the primary concerns we often see in discussions with our clients, is the level of trust we can reasonably place in an AI to explain legal and medical risks.
If an automated agent provides a simplified explanation of a complex procedure, there is a risk that it might overlook a critical detail or provide an inaccurate interpretation of the law.
This creates a difficult balance for healthtech leaders who must decide how to weigh the speed of automation against the safety of human oversight.
Most current discussions suggest that while technology can handle the majority of the conversation, a human clinician must still remain involved to verify high-risk decisions and ensure that the patient is truly informed.
Overcoming technical integration barriers in clinical workflow automation
Technical integration presents another significant hurdle, often referred to as the last mile of connectivity.
Even with the widespread adoption of modern data standards like the latest version of FHIR, many hospitals and clinics still operate on older electronic health record systems that were not built to communicate with modern web services.
This creates a disconnect between a sleek, automated consent platform and the rigid, older databases where patient information is actually stored.
Technical officers at healthcare firms are currently debating whether to build temporary bridges or wait for legacy providers, as these infrastructure gaps remain a primary hurdle for scaling clinical workflow automation.
For now, the difficulty of making different generations of technology work together remains a constant friction point for anyone trying to scale clinical workflow automation.
Navigating a fragmented global regulatory landscape
Finally, the landscape of global regulations is becoming increasingly fragmented. HIPAA, GDPR, PIPL, PIPEDA, LGPD… you name it.
There is no single set of rules for data privacy, as different regions continue to develop their own strict requirements.
For example, a company operating internationally must navigate the European Union’s laws on AI alongside various state privacy acts in the United States and new data protection rules in India.
These laws often require that a patient’s health information stay within the borders of their own country, a concept known as data residency. This makes it much harder to build a single, unified service that works everywhere.
The EU AI Act: moving beyond data protection
One of the most significant shifts in 2026 is the full enforcement of the EU AI Act, which marks a transition from simple data protection to a rigorous product certification model. Unlike the GDPR, which focuses on how personal data is processed, the AI Act classifies many healthcare applications (including AI-driven clinical decision support and triage tools) as high-risk systems.
- Pre-market certification: High-risk AI systems must now undergo a mandatory pre-market conformity assessment and receive a CE marking before they can be deployed in the European market.
- Strict governance standards: Providers are required to implement continuous risk management, maintain high-quality data governance, and ensure a high level of accuracy and cybersecurity throughout the system’s lifecycle.
- Human oversight: The law mandates that AI systems used in clinical environments include reinforced controls for human supervision to prevent automation bias and ensure patient safety.
To succeed in this environment, a consent service must be intelligent enough to handle different legal rules for every user while still providing a smooth and simple experience for the patient.
The future is automated
As we look toward the future healthcare landscape, it is clear that the traditional way of handling patient permissions is no longer sustainable. Consent should no longer be viewed as a static stop sign at the beginning of a clinical journey. Instead, it must become a dynamic engine that powers the entire workflow.
Consent-as-a-Service is the key to unlocking true interoperability, protecting patient rights, and restoring clinical time to what matters most: the patient.
The technology exists, the standards (FHIR, USCDI) are mature, and the patient demand is undeniable.
Ultimately, the future of clinical workflow automation depends on the ability to bridge the gap between complex legal requirements and the patient experience. The most successful organizations in 2026 will be those that recognize consent is more than just a signature on a page.
We specialize in building custom, FHIR-native smart consent layers that integrate seamlessly with your existing EHR.Whether you are looking to automate complex legal intake or implement AI-driven clinical triage, our team has the domain expertise to accelerate your clinical workflow automation roadmap.