Updated in Jan 2026
✏️ Summary
If you only have 2 minutes: The 2026 clinical landscape has shifted from AI-assisted (tools) to AI-first (autonomous agents).
In 2026, healthcare is transitioning toward an AI-powered clinical workflow paradigm, moving beyond simple AI assistance to truly autonomous care operations that fundamentally reimagine care delivery. This shift is powered by the convergence of ambient clinical intelligence, agentic AI for workflow orchestration, and advanced computer vision.
These systems actively manage routine tasks: such as real-time documentation, prior authorization, and care coordination, without requiring constant human intervention. By shifting the human role from administrative data entry to high-value clinical oversight, AI-first workflows allow technology to adapt to clinicians rather than forcing clinicians to adapt to rigid system constraints.
Healthcare in 2026 stands at an important turning point. Sounds cliché – we know – but still, it’s the truth.
After decades of digitization efforts that promised efficiency but delivered complexity, a new paradigm is emerging: AI-powered clinical workflows.
Unlike traditional systems where artificial intelligence serves as an add-on to existing processes, AI-first workflows position intelligent automation at the foundation of care delivery. It has been fundamentally reimagining how clinicians interact with technology, documentation, and patients.
In 2026, we’re witnessing the transition from AI-assisted healthcare to truly autonomous care operations.
These systems don’t just suggest actions or assist with documentation; they actively orchestrate clinical workflows, anticipate needs, and execute routine tasks without human intervention.
The result is a dramatic reduction in administrative burden and clinical burnouts, with early adopters reporting 60-75% decreases in documentation time and measurable improvements in both clinician satisfaction and patient outcomes.
This shift is powered by 3 converging technologies: ambient clinical intelligence that captures and structures clinical encounters in real-time, agentic AI systems capable of autonomous decision-making and task execution, and advanced computer vision that augments diagnostic capabilities.
This comprehensive guide explores where autonomous care operations begin, how to implement AI-powered workflows in your organization, and what the future holds as healthcare moves from experimentation to widespread adoption of intelligent automation.
Table of content
Introduction: The 2026 Clinical Crisis and the AI-First Musthave
Chapter 1: Beyond assisted AI, the rise of Clinical Autonomy
Chapter 2: The 4 engines of the autonomous workflow
Chapter 3: Implementation strategy for CTO/tech leaders
Chapter 4: Calculating ROI—The Hard Metrics of 2026
Chapter 5: Risks, Governance, and Ethics
Conclusion
Chapter 1: The Pre-AI Documentation Burden That’s Breaking Healthcare
Every year, physicians spend an estimated 4.5 hours per day on electronic health record (EHR) documentation and administrative tasks — nearly as much time as they spend with patients.
This crushing administrative burden has become the primary driver of physician burnout, with over 62% of U.S. physicians reporting symptoms of burnout in recent surveys (source).
The consequences extend far beyond clinician well-being: documentation overload contributes to diagnostic errors, delayed care, reduced patient satisfaction, and an estimated $4.6 billion annually in turnover costs from physicians leaving practice.
The promise that technology broke
The digitization of healthcare, accelerated by meaningful use requirements and EHR mandates, promised to streamline workflows and improve care quality. Instead, it created a new category of problems.
Clinicians became data entry clerks, spending evenings and weekends completing “pajama time” charting. The average physician clicks a mouse 4,000 times during a 10-hour shift. Patient interactions became mediated through screens, with doctors typing furiously while trying to maintain eye contact.
Traditional EHR systems were built on a document-centric, human-input model that simply digitized paper processes without reimagining workflows for the digital age.
Each new feature added complexity. Integration between systems remained fragmented. And despite billions invested in healthcare IT infrastructure, the fundamental inefficiency persisted: humans doing work that machines could handle better.
Why AI assistance isn’t enough (surprising?)
The first wave of healthcare AI focused on assistance: ambient listening tools that generate notes, algorithms that flag abnormal results, chatbots that answer patient questions.
These tools helped at the margins but didn’t fundamentally change workflows. Clinicians still reviewed, edited, and signed every note. They still responded to every alert. They still orchestrated every aspect of care coordination manually.
✏️ Our observation: AI assistance treats the symptom but not the disease.
It optimizes broken workflows rather than reimagining them. True transformation requires moving beyond assistance to autonomy—to systems that don’t just help humans work faster but actually take over entire categories of cognitive work.
The AI-first imperative
Enter AI-powered clinical workflows: systems designed from the ground up with intelligent automation at their core.
These workflows recognize that the optimal way to deliver care isn’t to make humans better at using computers, but to make computers better at supporting human expertise. They shift the human role from data entry and task execution to oversight, exception handling, and high-value clinical reasoning that machines cannot replicate.
This isn’t a distant future vision. Leading health systems are already implementing AI-first workflows in which autonomous agents handle appointment scheduling, prior authorization, care gap closure, and follow-up coordination.
Ambient intelligence captures clinical encounters without clinician input. Computer vision systems assess wounds, analyze imaging, and monitor vital signs continuously. The technology exists.
Defining: AI-Powered vs. AI-Assisted
The distinction between AI-first and AI-assisted workflows is more than semantic—it represents a fundamental philosophical difference in how technology supports clinical care.
AI-assisted workflows augment existing processes. A physician dictates a note, and AI helps clean up the grammar. An algorithm flags a suspicious finding, but a radiologist makes the final call. A chatbot answers routine patient questions, but complex queries get escalated to staff. The human remains the primary actor, with AI serving as a helpful tool.
AI-powered workflows invert this relationship. Intelligent systems handle entire processes end-to-end, with humans providing oversight and handling exceptions.
The clinical encounter happens naturally, and a complete, coded note appears in the EHR without clinician input. An agent autonomously coordinates specialist referrals, checking insurance eligibility, finding appointments, and communicating with patients.
Computer vision continuously monitors wounds, automatically documenting healing progression and alerting clinicians only when intervention is needed.
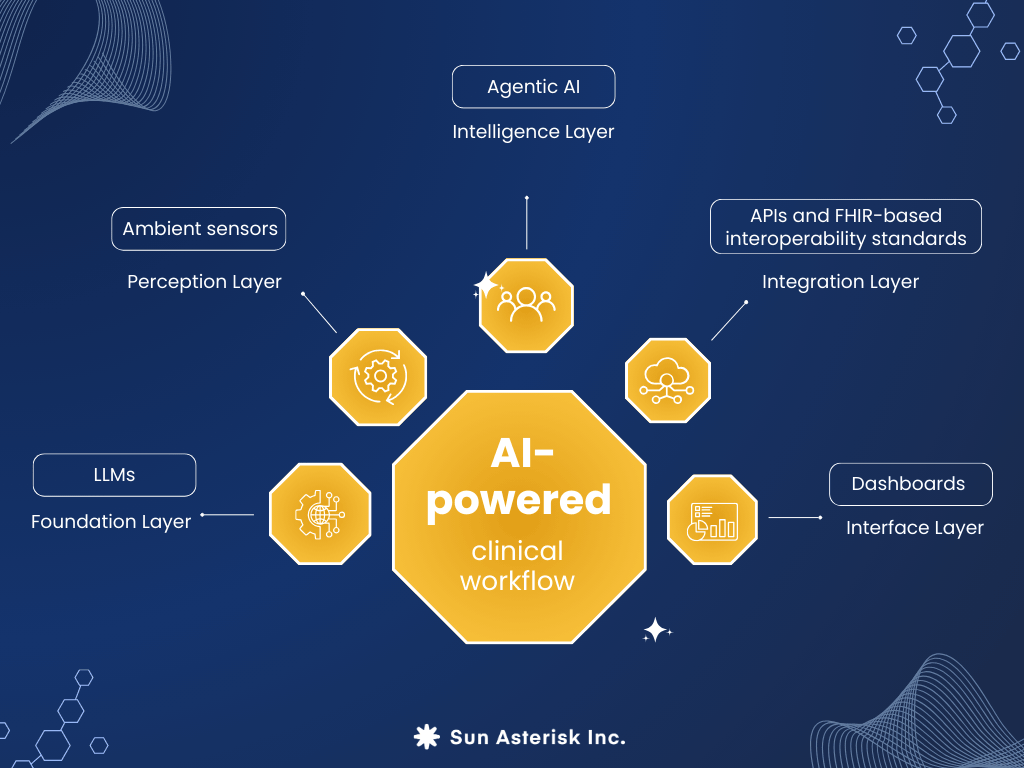
Core components and architecture
An AI-powered clinical workflow comprises several integrated layers:
Foundation Layer: Large language models (LLMs) trained on medical literature, clinical notes, and conversational data provide natural language understanding, generation, and reasoning capabilities. These models understand medical terminology, clinical logic, and documentation requirements.
Perception Layer: Ambient sensors (microphones, cameras, wearables) continuously capture clinical data—conversations, images, vital signs, movement patterns. Advanced speech recognition and computer vision transform this raw data into structured information.
Intelligence Layer: Agentic AI systems process structured information, make decisions, and orchestrate actions across multiple systems. These agents understand context, maintain memory across interactions, and can plan multi-step workflows to achieve objectives.
Integration Layer: APIs and FHIR-based interoperability standards enable seamless communication between AI systems and existing healthcare IT infrastructure—EHRs, PACS, laboratory systems, scheduling platforms, billing systems.
Interface Layer: Intuitive dashboards provide clinicians visibility into autonomous processes, allowing oversight and intervention when needed. Exception queues surface items requiring human judgment.
 The role of Agentic AI systems
The role of Agentic AI systems
The concept of “agentic AI” represents a crucial evolution beyond traditional automation. Rather than following rigid, predefined rules, agentic systems can:
- Set and pursue goals: Given a high-level objective (“ensure this patient receives appropriate follow-up care”), an agent can determine the necessary steps and execute them
- Maintain context and memory: Agents remember previous interactions, understand patient history, and make decisions based on accumulated context
- Make autonomous decisions: Within defined guardrails, agents can choose between options without human approval for each decision
- Coordinate across systems: Agents interact with multiple platforms, APIs, and data sources to accomplish complex workflows
- Learn and adapt: Through feedback loops, agents improve performance over time
In healthcare, agentic AI manifests as virtual care coordinators that manage population health outreach, documentation assistants that create complete notes from ambient data, and scheduling agents that optimize appointment books while considering clinical appropriateness, patient preferences, and resource constraints.
Real-time vs. Retrospective processing
A defining characteristic of AI-first workflows is their emphasis on real-time processing.
Traditional documentation workflows are retrospective: the clinical encounter happens, then the clinician spends time afterward documenting what occurred. This creates delay, introduces recall errors, and consumes valuable time.
AI-first workflows operate in real-time:
- Ambient intelligence captures and structures information as the encounter unfolds
- Clinical decision support triggers during the conversation, not hours later during chart review
- Documentation appears complete and coded by the end of the visit
- Orders, prescriptions, and referrals generate automatically based on clinical decisions made during the encounter
This real-time capability doesn’t just save time but it fundamentally changes the quality of care.
Clinicians can review and validate documentation while the patient is still present, catching errors immediately. Patients leave with complete visit summaries and clear next steps. Care coordination begins the moment decisions are made, not days later when someone processes a work queue.
Chapter 2: The Engines of the Autonomous Workflow
The Paradigm Shift: Technology That Adapts to Clinicians
Perhaps the most profound aspect of AI-first workflows is the reversal of adaptation burden. For decades, healthcare forced clinicians to adapt to technology – learning complex EHR interfaces, conforming to rigid documentation templates, interrupting conversations to enter data, modifying natural clinical workflows to fit system constraints.
AI-first workflows flip this dynamic. Technology adapts to clinicians:
- Natural conversation replaces template completion
- Ambient capture eliminates data entry
- Intelligent agents handle routine coordination tasks
- Interfaces surface relevant information proactively rather than requiring navigation through menus
This shift recognizes a fundamental truth: clinician expertise and patient relationships are the core value in healthcare.
Technology exists to support that core value, not to extract data from it. When systems adapt to clinicians rather than vice versa, satisfaction increases, burnout decreases, and care quality improves—precisely the outcomes that eluded traditional healthcare IT implementations.
3 pillars of AI-powered clinical operations
The transition to AI-first clinical operations rests on three foundational pillars, each representing a category of technology that has matured sufficiently for production deployment. Together, these pillars create a comprehensive ecosystem where autonomous systems handle the majority of cognitive work in healthcare delivery.
1, Ambient Clinical Intelligence
 The end of manual documentation
The end of manual documentation
Ambient clinical intelligence (ACI) represents the most immediately transformative technology in AI-first workflows. These systems use advanced speech recognition, natural language processing, and medical knowledge graphs to capture clinical encounters passively and generate comprehensive documentation automatically.
The technology works deceptively simply: microphones in the exam room capture the natural conversation between clinician and patient.
AI models trained on millions of clinical encounters transcribe the conversation, identify clinically relevant information, understand context and medical nuances, and structure findings into proper documentation format.
Within seconds of the encounter ending, a complete SOAP note appears in the EHR, properly coded with ICD-10 diagnoses and CPT procedures, ready for clinician review and signature.
Beyond transcription: Clinical intelligence
Early ambient documentation tools functioned essentially as intelligent transcription services: they captured what was said and formatted it nicely.
Modern ACI systems demonstrate true clinical intelligence:
- Medical reasoning: Understanding that “the patient’s leg is bothering her again” likely refers to a previously documented condition and automatically linking to relevant history
- Clinical inference: Recognizing that documented symptoms warrant specific preventive screenings or care gap closure opportunities
- Quality measure capture: Automatically identifying and documenting quality metrics, social determinants of health, and reportable data elements
- Contextual adaptation: Tailoring documentation specificity based on visit type, clinical specialty, and regulatory requirements
This intelligence transforms documentation from a compliance burden into a clinical tool that actually enhances care.
Integration and workflow optimization
The true power of ambient intelligence emerges through deep EHR integration. Rather than generating notes that clinicians must copy-paste into records, modern ACI systems:
- Write directly to discrete EHR fields, not just note sections
- Trigger clinical decision support rules based on documented findings
- Auto-populate order sets based on documented clinical decisions
- Generate patient instructions and after-visit summaries automatically
- Update problem lists, medication reconciliation, and care plans
This integration means that a 15-minute patient encounter requires perhaps 2 minutes of clinician time for review and validation rather than 10+ minutes of documentation time.
The time savings compound across dozens of daily encounters, giving physicians back hours each day for patient care, clinical reasoning, or simply ending their workday at a reasonable hour.
Real-world impact
Healthcare organizations implementing ambient clinical intelligence report remarkable outcomes.
Documentation time decreases by 60-75%. Physician satisfaction scores increase significantly. Patient satisfaction improves as clinicians maintain eye contact rather than typing.
Chart closure rates improve dramatically: for example, notes signed within 24 hours rather than days or weeks later.
And perhaps most importantly, clinicians consistently report that ambient documentation captures clinical nuances and patient voice more completely than their rushed manual notes ever did.
2, Agentic AI for Workflow Orchestration
 From task automation to autonomous coordination
From task automation to autonomous coordination
While ambient intelligence handles documentation, agentic AI tackles the broader challenge of healthcare’s countless coordination tasks: appointment scheduling, prior authorization, care gap closure, prescription refills, test result follow-up, and dozens of other workflows that consume staff time and delay care.
Agentic AI systems operate as virtual team members with defined roles and responsibilities.
Unlike traditional automation that follows rigid if-then rules, these agents can:
- Understand complex, multi-step workflows
- Make contextual decisions based on patient specifics
- Communicate with patients, staff, and external systems
- Adapt to exceptions and unexpected situations
- Learn from feedback to improve performance
Multi-agent healthcare ecosystems
The most sophisticated implementations deploy multiple specialized agents working collaboratively:
Care Coordination Agent: Manages referrals by checking insurance eligibility, finding in-network specialists with available appointments, sending referral documentation, scheduling appointments, and following up to ensure patients attend. This agent replaces hours of staff phone tag with autonomous coordination that completes in minutes.
Prior Authorization Agent: Processes authorization requests by gathering required clinical documentation, submitting requests through payer portals, monitoring status, responding to requests for additional information, and escalating denials for peer-to-peer review. What previously took days and multiple staff touches happens autonomously overnight.
Population Health Agent: Identifies care gaps based on quality measures and clinical guidelines, stratifies patients by risk, autonomously reaches out via preferred communication channels (text, email, phone), schedules appropriate appointments, and documents outreach efforts. A single agent can manage population health for thousands of patients.
Prescription Management Agent: Processes refill requests, checks drug interactions and contraindications, obtains prior authorization when needed, sends prescriptions to preferred pharmacies, and follows up on non-adherence. Routine refills happen without clinician involvement.
Results Follow-Up Agent: Monitors lab and imaging results, applies clinical protocols to determine which results require immediate attention, generates patient-friendly explanations, communicates results through patient portals, schedules follow-up appointments when indicated, and escalates concerning findings to clinicians.
Decision-making capabilities and guardrails
The key to effective agentic AI lies in striking the right balance between autonomy and oversight.
Agents must have sufficient decision-making authority to operate efficiently but must escalate situations requiring human judgment.
Well-designed healthcare agents operate within clearly defined guardrails:
- Clinical protocols: Agents follow evidence-based protocols and organizational policies
- Risk stratification: High-risk situations automatically escalate to clinical staff
- Preference learning: Agents learn individual clinician preferences and adapt behavior accordingly
- Audit trails: Complete logging enables review of agent decisions and actions
- Confidence thresholds: When agents are uncertain, they request human input rather than guessing
This approach allows agents to handle 80-90% of routine workflows autonomously while ensuring appropriate human oversight for complex situations.
The compound effect on efficiency
The impact of agentic AI compounds as multiple agents work in concert. Consider a patient diagnosed with diabetes during a primary care visit:
- Ambient intelligence documents the diagnosis and generates orders for HbA1c, lipid panel, and ophthalmology referral
- The care coordination agent schedules lab work at a convenient location, finds an in-network ophthalmologist, and books an appointment
- The patient education agent sends personalized diabetes education materials and nutrition resources
- The population health agent enrolls the patient in diabetes management programming and schedules follow-up appointments
- The prescription agent processes orders for metformin and statins, checking formulary coverage and obtaining prior authorization if needed
- When lab results return, the results agent communicates findings to the patient and schedules follow-up based on values
This entire care coordination sequence which would traditionally require hours of staff time spread across days or weeks – happens autonomously within hours.
The patient experiences seamless, coordinated care. Staff focus on complex cases requiring human judgment. And clinicians retain oversight without drowning in routine coordination tasks.
3, Computer Vision & Diagnostic Augmentation
 Visual intelligence in clinical workflows
Visual intelligence in clinical workflows
Computer vision represents the third pillar of AI-first workflows, bringing autonomous capabilities to visual assessment tasks that have traditionally required human observation and documentation.
Modern computer vision systems in healthcare leverage deep learning models trained on millions of clinical images to perform sophisticated visual analysis:
- Wound assessment: Automated measurement of wound dimensions, depth, and tissue type, with photographic documentation and healing trajectory tracking
- Vital signs monitoring: Contactless measurement of heart rate, respiratory rate, and oxygen saturation through video analysis
- Skin condition documentation: Objective assessment and classification of rashes, lesions, and dermatological conditions
- Mobility monitoring: Fall risk assessment through gait analysis and movement pattern recognition
- Nutritional monitoring: Food intake tracking and portion estimation in institutional settings
Pattern recognition in medical imaging
While diagnostic radiology represents the most publicized application of AI vision, practical implementation in AI-first workflows focuses on augmentation rather than replacement:
Triage and prioritization: AI reviews imaging studies as they’re completed, flagging critical findings (intracranial hemorrhage, pneumothorax, fractures) for immediate radiologist attention. This ensures urgent cases receive priority without requiring radiologists to monitor every incoming study.
Workflow optimization: Computer vision pre-processes studies by identifying anatomy, measuring structures, and generating preliminary observations. Radiologists review AI-enhanced studies rather than starting from scratch, improving efficiency and consistency.
Quality assurance: AI performs secondary reads to catch potential oversights, flagging discrepancies between radiologist interpretation and algorithmic assessment for double-checking.
Continuous monitoring applications
Perhaps the most transformative application of computer vision in AI-first workflows is continuous, passive monitoring:
- Post-surgical wound monitoring: Patients photograph wounds daily using smartphones. Computer vision automatically assesses healing, measures wound dimensions, detects signs of infection, and alerts clinicians to complications. Routine healing requires no clinical time; only problems trigger human review.
- Chronic condition tracking: Diabetic patients photograph their feet weekly. AI assesses for ulcers, inflammation, and structural changes, enabling early intervention before serious complications develop.
- Remote patient monitoring: Video monitoring in skilled nursing facilities detects falls, monitors movement patterns for functional decline, and alerts staff to changes requiring attention—all without constant human observation.
This continuous monitoring paradigm prevents problems rather than reacting to them, reduces unnecessary clinical visits, and catches complications earlier when they’re easier to treat.
Integration with clinical workflows
Computer vision capabilities integrate seamlessly into AI-first workflows:
- Images captured during encounters automatically import into EHRs
- Measurements and assessments populate discrete data fields for trending and analysis
- Abnormal findings trigger clinical decision support alerts
- Agentic AI systems use visual data to inform care coordination decisions
- Longitudinal image comparison enables objective assessment of progression or improvement
The result is comprehensive documentation of visual findings without adding burden to clinical workflows—the system sees, assesses, documents, and alerts autonomously.
Chapter 3: Implementation Strategy for Healthtech Leaders
Foundation models and healthcare LLMs
At the core of AI-first clinical workflows are large language models (LLMs) specifically adapted for healthcare applications. These foundation models represent a quantum leap beyond earlier natural language processing systems:
General-purpose foundation models like GPT-4, Claude, and Gemini provide broad language understanding, reasoning, and generation capabilities. They excel at conversational interaction, complex reasoning tasks, and generating human-quality text.
Healthcare-specialized LLMs build on these foundations with additional training on medical literature, clinical notes, drug databases, diagnostic criteria, and treatment guidelines. Models like Google’s Med-PaLM 2, Microsoft’s BioGPT, and various proprietary clinical LLMs developed by EHR vendors understand medical terminology, clinical reasoning patterns, and healthcare-specific contexts that general models miss.
The specialization matters. A general LLM might generate grammatically correct but clinically nonsensical documentation.
Healthcare LLMs understand that “SOB” means shortness of breath, not a profane insult.
They know that vital signs outside normal ranges warrant clinical attention. They grasp the difference between a medication order and a discontinued medication in the context of a clinical conversation.
FHIR and interoperability standards
The promise of AI-first workflows depends entirely on interoperability—the ability of diverse systems to exchange and use clinical information. Fast Healthcare Interoperability Resources (FHIR) has emerged as the critical standard enabling this exchange.
FHIR basics: Rather than the monolithic data structures of previous standards (HL7 v2, CDA), FHIR defines modular “resources” representing clinical concepts—patients, encounters, medications, observations, conditions. These resources use modern web standards (REST APIs, JSON, OAuth) familiar to developers, dramatically reducing integration complexity.
💡Why FHIR enables AI-first workflows:
- Standardized data access: AI agents can retrieve patient data from any FHIR-compliant system using the same query patterns
- Write-back capabilities: Agents can create or update clinical data in EHRs without custom integration for each vendor
- Real-time integration: FHIR supports subscriptions, allowing AI systems to receive notifications of clinical events as they occur
- Granular permissions: Fine-grained authorization ensures AI systems access only appropriate data
Major EHR vendors now expose FHIR APIs, and CMS regulations mandate patient data access through FHIR. This standardization means that AI-first workflow solutions can integrate with diverse health systems without rebuilding custom interfaces for each organization.
Edge computing and data privacy
Healthcare’s stringent privacy requirements and need for real-time responsiveness drive architectural decisions around where AI computation happens:
Edge computing architecture processes sensitive data locally rather than transmitting it to cloud servers:
- Ambient intelligence devices perform speech recognition and preliminary processing on-premises
- Computer vision analysis happens on local servers or edge devices
- Only structured, de-identified data transmits to cloud-based AI services
- Critical real-time decisions process locally even if network connectivity fails
Privacy-preserving techniques enable AI capabilities while protecting patient data:
- Federated learning: AI models train across multiple institutions without sharing raw patient data
- Differential privacy: Statistical noise added to datasets prevents identification of individual patients while preserving analytical utility
- Homomorphic encryption: Computations performed on encrypted data without decryption
- Secure enclaves: Trusted execution environments process sensitive data with hardware-enforced isolation
These techniques aren’t theoretical.
Production AI-first workflow systems deploy them today to meet HIPAA requirements and organizational security policies.
Integration architecture
Successful AI-first implementations require thoughtful integration architecture that balances multiple concerns:
Layered approach: Modern healthcare integration follows a layered pattern:
- Data foundation layer: EHRs, PACS, lab systems, and other source systems
- Integration layer: FHIR servers, HL7 interfaces, and data normalization services that translate between different data formats
- Intelligence layer: AI/ML models, agentic AI orchestration, and decision engines
- Application layer: User interfaces, dashboards, and workflow tools that surface AI capabilities to clinicians and staff
Event-driven architecture: Rather than batch processing, AI-first systems react to clinical events in real-time:
- Patient check-in triggers ambient intelligence activation
- Lab result availability prompts results agent review
- Medication order entry triggers drug interaction checking
- Appointment scheduling initiates care coordination workflows
Microservices pattern: Rather than monolithic applications, capabilities deploy as independent services:
- Ambient documentation service
- Prior authorization agent
- Care gap identification service
- Clinical decision support engine
This modularity enables organizations to adopt AI-first capabilities incrementally, replacing or augmenting specific workflows without wholesale system replacement.
Scalability and performance considerations
AI-first workflows must operate at enterprise scale with reliability equivalent to mission-critical clinical systems:
Computational demands: Healthcare AI requires significant processing power:
- Real-time speech recognition and NLP for ambient intelligence
- Complex reasoning for agentic decision-making
- Image analysis for computer vision applications
- Simultaneous processing for dozens or hundreds of concurrent users
Modern implementations leverage cloud infrastructure that scales dynamically, provisioning resources based on demand. GPU-accelerated computing handles computationally intensive tasks like medical imaging analysis.
Latency requirements: Clinical workflows demand near-instantaneous response:
- Ambient documentation must complete within seconds of encounter ending
- Clinical decision support must trigger immediately when relevant conditions appear
- Computer vision analysis should process faster than manual assessment
Edge computing, model optimization, and intelligent caching ensure AI-first systems meet these performance expectations.
Reliability and fault tolerance: Healthcare systems cannot go down. AI-first architectures incorporate:
- Redundancy across multiple availability zones
- Graceful degradation when services fail
- Automatic failover to backup systems
- Human oversight queues when AI confidence is low
The technology stack behind AI-first clinical workflows represents a sophisticated convergence of advances in machine learning, software engineering, healthcare interoperability, and distributed systems.
→ For healthcare leaders, understanding these architectural foundations helps evaluate vendors, plan implementations, and set realistic expectations about what’s possible in 2026 and beyond.
Implementation roadmap: from pilot to production
Successful adoption of AI-first clinical workflows requires systematic planning and phased implementation.
Organizations that rush deployment without adequate preparation risk user resistance, integration failures, and disappointing results. Those that follow a structured roadmap achieve faster time-to-value and broader adoption.
Phase 1: Assessment and readiness evaluation
Before implementing AI-first workflows, conduct honest evaluation across key dimensions:
Technical infrastructure readiness:
- Does your EHR expose FHIR APIs for integration?
- Is network infrastructure adequate for real-time AI processing?
- Do exam rooms have reliable audio capture capability?
- What is your current interoperability maturity?
Clinical workflow analysis:
- Which workflows create the most administrative burden?
- Where do clinicians spend the most non-clinical time?
- What documentation backlogs exist?
- Which coordination tasks consume the most staff resources?
Change readiness:
- How do clinicians and staff view AI adoption?
- What is the history of technology implementation success?
- Are clinical champions available to lead adoption?
- What concerns or resistance exists?
Regulatory and compliance landscape:
- What approval processes govern new technology adoption?
- How does your risk management framework address AI?
- What vendor security and privacy requirements must be met?
- Are there specific regulatory constraints (state licensing, payer contracts)?
Use case prioritization
Not all AI-first workflows deliver equal value or face equal implementation challenges. Prioritize initial use cases based on:
High-impact, lower-complexity opportunities:
- Ambient clinical documentation in outpatient primary care
- Automated prior authorization for common procedures
- Results follow-up for routine lab tests
- Care gap identification and outreach
Success criteria definition: For each prioritized use case, define specific, measurable outcomes:
- Documentation time reduction targets
- Chart closure timeframe improvements
- Patient satisfaction score changes
- Staff time savings quantification
- Return on investment thresholds
Vendor selection and partnership
The AI-first workflow market is rapidly evolving with diverse vendors offering specialized capabilities. Evaluation should consider:
Core capabilities:
- Does the solution address your prioritized use cases?
- What is the depth of healthcare-specific training and optimization?
- How does accuracy compare across diverse clinical scenarios?
- What level of EHR integration is available?
Implementation support:
- What professional services support onboarding?
- How extensive is training and change management support?
- What is the typical implementation timeline?
- What post-deployment optimization is included?
Total cost of ownership:
- Licensing models (per-provider, per-encounter, platform fees)
- Implementation and integration costs
- Ongoing support and maintenance fees
- Infrastructure requirements
Strategic fit:
- Is the vendor financially stable with long-term viability?
- What is their product roadmap and innovation trajectory?
- Do they have proven healthcare implementations?
- How do they handle data ownership and portability?
Phase 2: Pilot Implementation
Pilot Scope Definition
Successful pilots balance sufficient scale to demonstrate value with controlled scope to manage risk:
Recommended pilot parameters:
- 10-20 clinicians across 2-3 clinical sites
- Single specialty or clinical department (often primary care)
- 3-month pilot duration
- Clear go/no-go decision criteria
Infrastructure preparation:
- Install required hardware (microphones, edge computing devices)
- Configure EHR integrations and test data flows
- Establish secure network connectivity
- Set up monitoring and logging infrastructure
Training and Onboarding
Clinician adoption depends on effective training that goes beyond technical operation:
Pre-deployment training (2-3 hours per clinician):
- How AI-first workflows differ from current processes
- Hands-on practice with ambient documentation review and validation
- Understanding AI capabilities and limitations
- Exception handling and escalation procedures
Ongoing support during pilot:
- On-site support staff during initial weeks
- Daily stand-up meetings to address issues
- Rapid iteration based on user feedback
- Documentation of lessons learned
Optimization and refinement: AI-first systems improve through feedback. Pilot phase focuses on:
- Reviewing documentation accuracy and completeness
- Adjusting ambient intelligence prompts and templates
- Refining agent decision-making thresholds
- Optimizing integration workflows
Measuring Pilot Success
Rigorous measurement during pilot phase informs expansion decisions:
Quantitative metrics:
- Documentation time per encounter (pre/post comparison)
- Chart closure rates and turnaround time
- Note quality scores (completeness, accuracy, billing capture)
- Patient satisfaction scores
- Clinician satisfaction and perceived burden
- Technical performance (uptime, latency, error rates)
Qualitative feedback:
- Structured clinician interviews at pilot conclusion
- Staff focus groups on workflow changes
- Patient feedback on encounter experience
- IT and compliance stakeholder perspectives
Go/no-go decision framework: Define clear success thresholds before pilot begins:
- Minimum documentation time savings (typically 50%+)
- Clinician satisfaction improvement targets
- Acceptable error rates and quality standards
- ROI validation showing positive return within defined period
Phase 3: Phased Expansion
Expansion Strategy
With successful pilot validation, systematic expansion balances speed with risk management:
Wave-based rollout approach:
- Wave 1 (Months 6-7): Expand within pilot specialty to additional sites
- Wave 2 (Months 8-9): Add second specialty or clinical area
- Wave 3 (Months 10-12): Broader organizational deployment
Site readiness criteria: Before expanding to new sites, ensure:
- Technical infrastructure meets requirements
- Clinical leadership buy-in secured
- Local champions identified and trained
- Support resources available for onboarding
Change Management and Adoption
Scaling beyond early adopters requires proactive change management:
Communication strategy:
- Share pilot results and success stories
- Address concerns and resistance transparently
- Establish clear expectations about timelines and support
- Celebrate wins and recognize champions
Training at scale:
- Transition from in-person to blended learning models
- Develop self-service resources and documentation
- Create peer learning and support networks
- Establish super-user programs for ongoing support
Addressing resistance: Not all clinicians embrace AI-first workflows immediately:
- Allow voluntary adoption initially to build positive word-of-mouth
- Provide extended training and support for reluctant users
- Address specific concerns with customization when possible
- Have clinical leaders model adoption and enthusiasm
Phase 4: Optimization and Expansion (ongoing)
Continuous Improvement
AI-first workflows improve continuously through:
Performance monitoring:
- Dashboards tracking key metrics across organization
- Quality reviews identifying improvement opportunities
- User feedback collection and analysis
- Benchmark comparison across sites and clinicians
Model refinement:
- Fine-tuning AI models based on organizational patterns
- Expanding agent capabilities based on proven success
- Adjusting automation thresholds based on error analysis
- Incorporating new features from vendor product roadmap
Expanding scope: With core workflows stabilized, expand to additional use cases:
- Additional clinical specialties
- Inpatient and emergency department workflows
- Complex care coordination scenarios
- Integration with telehealth and remote monitoring
Chapter 4: Calculating ROI—The Hard Metrics
Measuring Long-Term ROI
Demonstrate ongoing value through comprehensive ROI analysis:
Cost savings quantification:
- Clinician time savings (hours reclaimed × hourly compensation)
- Staff efficiency improvements (FTE equivalents)
- Reduced locum tenens and overtime costs
- Billing capture improvements and coding accuracy
Quality and satisfaction improvements:
- Physician burnout reduction and retention
- Patient satisfaction and experience scores
- Documentation quality improvements
- Compliance and quality measure capture
Strategic benefits:
- Enhanced ability to recruit clinicians
- Competitive differentiation in market
- Capacity expansion without headcount growth
- Platform for future innovation
Building Organizational AI Capabilities
The most successful organizations view AI-first workflow adoption as the foundation for broader AI transformation:
Data infrastructure development: AI-first systems generate rich data enabling:
- Population health analytics
- Predictive modeling for resource planning
- Quality improvement insights
- Clinical research opportunities
Cultural transformation: Successful AI adoption shifts organizational culture:
- Comfort with AI-augmented decision-making
- Data-driven continuous improvement mindset
- Reduced resistance to technological change
- Foundation for future innovation adoption
The implementation roadmap from pilot to production requires patience, systematic planning, and commitment to change management.
Organizations that invest in thoughtful deployment see transformative returns. Those that cut corners face user resistance, disappointing results, and stalled initiatives.
In 2026, the winners are those who started methodical implementation in 2024-2025. This gives them time to work through challenges and achieve maturity before competitors catch up.
Real-World Impact: Use Cases and Results
The theoretical promise of AI-first workflows is compelling, but practical validation comes from real-world implementations. Across diverse care settings, early adopters are demonstrating measurable impact and uncovering lessons that inform best practices.
Lessons Learned Across Implementations
What works
- Start with high-volume, lower-complexity workflows: Organizations achieving fastest ROI focused initially on primary care documentation and routine coordination tasks before tackling complex specialty workflows.
- Invest in integration: Deep EHR integration delivers far more value than standalone tools. Organizations that ensured AI systems wrote directly to EHRs saw higher adoption than those requiring manual data transfer.
- Prioritize user experience: Clinician-friendly interfaces and minimal workflow disruption drive adoption. Systems requiring significant behavior change faced resistance regardless of potential benefits.
- Measure and communicate results: Regular reporting of time savings, efficiency gains, and satisfaction improvements maintains momentum and leadership support.
Common fails
- Underestimating change management: Technical implementation is often easier than driving adoption. Organizations that treated AI-first workflows as “just another IT project” struggled with user resistance.
- Insufficient training: Clinicians who received comprehensive training showed 3x higher satisfaction than those given only brief orientation.
- Premature scaling: Organizations that expanded before validating pilot success faced implementation challenges and user skepticism.
- Neglecting optimization: AI-first systems require ongoing refinement. Organizations that deployed and walked away saw declining performance over time.
The real-world impact of AI-first clinical workflows is no longer theoretical. Across diverse settings and specialties, organizations are demonstrating dramatic improvements in efficiency, clinician satisfaction, and patient experience. The question for healthcare leaders in 2026 is not whether AI-first workflows deliver value, but how quickly they can implement them to remain competitive.
Chapter 5: Risks, Governance, and Ethics
Challenges and considerations in AI-first clinical workflow
While AI-first clinical workflows offer transformative potential, successful implementation requires navigating complex regulatory, ethical, and practical challenges.
Organizations that address these considerations proactively position themselves for sustainable success.
Regulatory compliance and FDA considerations
Healthcare AI exists in a complex regulatory environment with overlapping federal and state jurisdiction:
- FDA regulation of clinical AI: The FDA regulates AI systems that meet the definition of medical devices—software that diagnoses, treats, prevents, or mitigates disease. The regulatory approach depends on risk classification:
- Class I (low risk): General wellness applications and administrative tools typically require minimal FDA oversight. Most ambient documentation and workflow coordination agents fall here.
- Class II (moderate risk): Clinical decision support systems and diagnostic aids often require 510(k) clearance demonstrating substantial equivalence to predicate devices. Computer vision systems for diagnostic purposes typically fall in this category.
- Class III (high risk): AI systems that significantly influence life-or-death decisions or replace clinician judgment face the highest scrutiny with premarket approval requirements.
- The AI/ML exception pathway: The FDA recognizes that AI systems improve continuously unlike traditional static software. Their proposed framework for AI/ML-based software as a medical device creates pathways for iterative updates without requiring full reauthorization for each model improvement.
Practical regulatory strategy
Organizations implementing AI-first workflows should:
- Classify systems appropriately: Work with regulatory counsel to determine whether specific AI capabilities constitute medical devices requiring FDA oversight. Many workflow automation and documentation tools fall outside FDA jurisdiction as administrative rather than clinical systems.
- Choose FDA-cleared vendors when required: For capabilities requiring FDA oversight (particularly diagnostic computer vision), selecting vendors with appropriate clearances simplifies compliance.
- Document clinical validation: Even for systems not requiring FDA clearance, maintain rigorous validation studies demonstrating accuracy, safety, and clinical appropriateness. This documentation supports both internal governance and potential regulatory inquiry.
- Establish clinical oversight: Implement medical device committees or AI governance bodies to review AI implementations, monitor performance, and ensure ongoing safety.
Data privacy and HIPAA compliance
Protected Health Information in AI Systems
AI-first workflows process vast quantities of protected health information (PHI), creating significant privacy obligations:
HIPAA requirements for AI vendors: Third-party AI vendors accessing PHI must sign Business Associate Agreements (BAAs) accepting responsibility for HIPAA compliance. Due diligence should verify:
- Appropriate technical safeguards (encryption, access controls, audit logging)
- Administrative safeguards (workforce training, incident response procedures)
- Physical safeguards for systems processing PHI
- Breach notification procedures
- Data use limitations and prohibitions on unauthorized disclosure
De-identification considerations: AI model training often benefits from large datasets. HIPAA permits use of de-identified data without patient authorization if organizations follow safe harbor or statistical de-identification standards.
Minimum necessary principle: AI systems should access only PHI necessary for their specific function. Overly broad data access creates unnecessary risk.
Data sovereignty and storage
Healthcare organizations must consider:
- Geographic data storage: Some state laws or organizational policies require PHI remain within U.S. borders or specific regions. Cloud-based AI systems should offer geographic storage guarantees.
- Data retention and deletion: Policies should address how long AI systems retain data and establish procedures for patient-requested deletion.
- Audit trails and transparency: Comprehensive logging of AI access to patient data supports HIPAA audit requirements and enables investigation of potential breaches.
The Bias challenge
AI systems trained on historical healthcare data risk perpetuating or amplifying existing disparities:
- Training data bias: If AI models train predominantly on data from specific populations, they may perform poorly for underrepresented groups. For example, ambient intelligence systems trained primarily on native English speakers may struggle with accented speech or multilingual conversations.
- Algorithmic bias: Even with diverse training data, AI decision-making may exhibit bias. Studies have documented racial bias in healthcare algorithms, including systems that systematically recommend different care levels for patients of different races with similar clinical profiles.
- Outcome disparities: Biased AI can worsen health equity if deployment patterns favor well-resourced organizations and populations while underserved communities lack access to beneficial technology.
Equity-focused implementation
Organizations committed to equitable AI deployment should:
- Assess algorithm fairness: Before deployment, evaluate AI performance across demographic groups (race, ethnicity, gender, age, language). Identify and address disparate performance.
- Monitor for bias continuously: Ongoing performance monitoring should stratify results by demographics, surfacing emerging bias issues.
- Ensure diverse representation: Include diverse clinical and patient perspectives in AI selection, configuration, and governance decisions.
- Prioritize access equity: Deploy AI-first workflows in safety-net settings and underserved populations, not only wealthy systems, to reduce rather than widen the digital divide.
- Support multiple languages: For patient-facing AI (care coordination agents, results communication), ensure support for languages spoken by patient populations.
Liability and accountability
Who’s responsible when AI fails to operate?
AI-first workflows create novel liability questions:
Clinical responsibility: Fundamentally, clinicians remain legally responsible for patient care. Delegation to AI systems doesn’t eliminate clinician accountability. This creates obligations to:
- Understand AI capabilities and limitations
- Provide appropriate oversight of autonomous systems
- Validate AI-generated information before relying on it for clinical decisions
- Intervene when AI recommendations appear inappropriate
Vendor liability: AI vendors typically limit liability through contractual disclaimers. Healthcare organizations should negotiate:
- Clear performance warranties and service level agreements
- Indemnification for vendor negligence or system failures
- Insurance coverage for AI-related incidents
- Transparent disclosure of known limitations
Institutional accountability: Healthcare organizations bear responsibility for:
- Appropriate AI vendor selection and due diligence
- Adequate training of clinicians using AI systems
- Ongoing monitoring of AI performance and safety
- Governance processes ensuring appropriate AI use
Risk mitigation strategies
- Graduated autonomy: Implement AI systems with appropriate human oversight. High-risk decisions should require human validation; low-risk routine tasks can be fully autonomous.
- Comprehensive documentation: Maintain detailed records of AI system configuration, decision logic, and performance monitoring to demonstrate due diligence in the event of adverse outcomes.
- Incident response procedures: Establish clear protocols for identifying, reporting, investigating, and addressing AI-related safety events.
- Professional liability insurance: Ensure malpractice insurance policies adequately cover AI-augmented care delivery. Some policies may require specific endorsements.
Transparency and explainability
Modern AI systems often function as “black boxes”: making accurate predictions through complex mathematical transformations that resist human interpretation. This creates challenges:
- Clinical trust: Clinicians hesitate to trust recommendations they can’t understand or validate through clinical reasoning.
- Regulatory expectations: FDA guidance increasingly emphasizes the importance of AI explainability, particularly for higher-risk applications.
- Patient rights: Patients have ethical and sometimes legal rights to understand how AI influences their care.
Consent and patient autonomy
Patient autonomy requires informed consent for significant care decisions. AI-first workflows raise questions about disclosure:
What patients should know:
- That AI systems assist in documentation and care coordination
- Which clinical decisions involve AI input
- How to access human clinicians when desired
- How AI uses their health information
Practical disclosure approaches:
- General notices in patient handbooks and intake materials
- Signage in exam rooms where ambient intelligence operates
- Specific consent for applications with higher patient impact (e.g., AI-driven diagnosis)
Opt-out considerations: Some organizations offer patients the ability to opt out of certain AI applications (particularly ambient documentation). This respects autonomy but creates workflow complexity.
Current AI in clinal adoption landscape
The AI-first clinical workflow market is experiencing rapid growth and transformation as we move through 2026:
Market penetration statistics: Industry analysis suggests that approximately 35-40% of U.S. health systems have deployed some form of ambient clinical intelligence, up from less than 5% in 2023 (source).
Adoption of agentic AI for care coordination remains earlier-stage at approximately 15-20% penetration, but growing at over 150% year-over-year.
Leading health systems: Major academic medical centers and large integrated delivery networks have emerged as early adopters, driven by:
- Greater resources for technology investment
- More acute clinician burnout and recruitment challenges
- Stronger technical capabilities for complex integration
- Research interests in AI innovation
Expansion to community hospitals and practices: A significant trend in 2026 is democratization beyond elite institutions. Smaller organizations are adopting AI-first workflows through:
- Simplified vendor solutions requiring less technical sophistication
- Cloud-based deployment models reducing infrastructure requirements
- Bundled solutions from major EHR vendors
- Group purchasing organizations negotiating favorable contracts
Geographic patterns: Adoption concentrates initially in competitive healthcare markets where clinician recruitment is challenging and patients have choices. Urban and suburban markets show higher penetration than rural areas, though this gap is narrowing as vendors develop solutions for resource-constrained settings.
To conclude: Time to take action
Healthcare leaders face a stark choice: lead the transformation or be disrupted by it.
Need AI-first workflow audit and strategy implementation support? Contact our experts!